EU Commission waters down GM regulation just as new research shows “safe” GM maize causes increased tumours, organ damage, and premature death
20 September 2012
The European Commission is pushing through a new Regulation
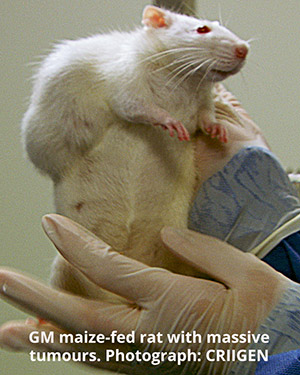 The Commission’s move comes just as shocking new research has revealed that a GM maize already approved in Europe for use in food and feed, and Roundup, the weedkiller used with it, can cause increased tumours, premature death and organ damage at levels claimed to be safe by EU regulatory authorities.[3]
The Commission’s move comes just as shocking new research has revealed that a GM maize already approved in Europe for use in food and feed, and Roundup, the weedkiller used with it, can cause increased tumours, premature death and organ damage at levels claimed to be safe by EU regulatory authorities.[3]
Claire Robinson, research director at Earth Open Source, said, “If adopted, the draft Regulation will leave the public even more exposed to serious health risks such as those revealed by the new study.”
Earth Open Source’s report says the draft Regulation undermines democratically established EU GMO legislation and betrays demands in 2008 by the EU Environment Council that the EU’s GMO safety assessment be strengthened.[4]
The draft Regulation has not been subjected to formal Parliamentary scrutiny but will be voted on in a behind-closed-doors Commission committee on an undisclosed date in the coming weeks. Earth Open Source believes that because of the untransparent way the draft Regulation is being progressed, it has passed ‘under the radar’ of the public, some NGOs, and member states.
Earth Open Source is calling on the Commission to freeze the progress of the draft Regulation and open it to full public consultation in light of the new scientific findings.
The new study found that rats fed over two years with GM maize NK603 or dosed with Roundup at levels permitted in drinking water, food and feed, died earlier than rats fed a non-GM diet. They developed massive tumours and liver and kidney damage. Even the lowest doses caused severe health problems.
Yet EU authorities had approved this GM maize as safe, based on a short 90-day rat feeding trial commissioned by the crop’s developer, Monsanto. The new research shows that rats only began to develop tumours after four months – an effect that the 90-day feeding trials typically done for GMO assessments cannot detect, as 90 days is just too short.
Worryingly, the draft Regulation does not require long-term feeding trials and contains problematic wording that could enable even the weak 90-day study to be waived in future. This would fit the stance of the European Food Safety Authority (EFSA), which has questioned the need for feeding trials. EFSA also claimed that 90-day trials are sufficient to identify long-term toxic effects.[5]
The new study shows that EFSA’s and the Commission’s positions are faulty. Clearly, feeding trials are necessary to reveal unexpected toxic effects. And they must be long-term. While 90-day trials failed to detect tumours and premature deaths, the longer 2-year study revealed this harm.
Earth Open Source’s report says EFSA must shoulder much of the responsibility for exposing European citizens and livestock to unsafe NK603 maize. A 2009 analysis by independent scientists of industry’s own 90-day trial data on NK603 maize showed that even this data revealed signs of liver and kidney toxicity.[6] But the European Food Safety Authority (EFSA) ignored these signs, concluding that NK603 was “as safe” as non-GM maize and “unlikely to have an adverse effect on human and animal health”.[7,8]
Claire Robinson said, “The history of this maize shows that EFSA is unfit for purpose and too close to the GM industry. EFSA’s industry-friendly stance on GMOs has unfortunately infected the draft Regulation.”
Weaknesses of the draft Regulation include:
- It makes the discredited concept of substantial equivalence or “comparative assessment” potentially the beginning and end of the risk assessment, taking the EU down the US route of almost non-existent GMO regulation. Virtually any GM crop could pass this weak assessment and escape being subjected to further rigorous tests, contradicting the democratically established GMO Regulation 1829/2003.
- It further weakens the comparative assessment by allowing irrelevant data to be introduced that mask the effects of GM, in contravention of EU Directive 2001/18 and good scientific practice.
Claire Robinson said, “The Commission must go back to the drawing board and revise this flawed Regulation to reflect current scientific knowledge, uphold European law, and fulfill the demands of the EU Environment Council. Otherwise we could all end up sharing the fate of the lab rats in the 2-year feeding trial.”
ENDS
Download the draft regulation here…
Notes
- European Commission (2012). Commission implementing regulation (EU) No…. on applications for authorisation of genetically modified food and feed in accordance with Regulation (EC) No 1829/2003 of the European Parliament and of the Council and amending Regulations (EC) No 641/2004 and (EC) No 1981/2006
- Earth Open Source (2012). EU Commission’s draft GMO Regulation: Charter for the GM industry. September. The report and supporting documents can be downloaded from this page.
- Séralini, G. E., E. Clair, et al. (2012). Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food and Chemical Toxicology.
- Environment Council of the European Union (2008). Genetically modified organisms – Council conclusions 16882/08. Brussels.
- European Food Safety Authority (EFSA) GMO Panel Working Group on Animal Feeding Trials (2008). Safety and nutritional assessment of GM plants and derived food and feed: The role of animal feeding trials. Food Chem Toxicol 46 (Suppl 1): S2-70.
- de Vendomois, J. S., F. Roullier, et al. (2009). A comparison of the effects of three GM corn varieties on mammalian health. Int J Biol Sci 5(7): 706–726.
- European Food Safety Authority (EFSA) (2003). Opinion of the Scientific Panel on Genetically Modified Organisms on a request from the Commission related to the safety of foods and food ingredients derived from herbicide-tolerant genetically modified maize NK603, for which a request for placing on the market was submitted under Article 4 of the Novel Food Regulation (EC) No 258/97 by Monsanto (QUESTION NO EFSA-Q-2003-002): Opinion adopted on 25 November 2003. EFSA Journal 2003(9): 1–14.
- European Food Safety Authority (EFSA) (2009). Applications (references EFSA-GMO-NL-2005-22, EFSA-GMO-RX-NK603) for the placing on the market of the genetically modified glyphosate tolerant maize NK603 for cultivation, food and feed uses, import and processing and for renewal of the authorisation of maize NK603 as existing products, both under Regulation (EC) No 1829/2003 from Monsanto: Scientific Opinion of the Panel on Genetically Modified Organisms (Questions No EFSA-Q-2005-249, No EFSA-Q-2008-075), Adopted on 27 May 2009. EFSA Journal 2009(1137): 1–50.
Some documents cited in Earth Open Source’s press release and report, “EU Commission’s draft GMO Regulation: Charter for the GM industry”:
- European Commission (2012). Commission implementing regulation (EU) No…. on applications for authorisation of genetically modified food and feed in accordance with Regulation (EC) No 1829/2003 of the European Parliament and of the Council and amending Regulations (EC) No 641/2004 and (EC) No 1981/2006.
- European Parliament and Council (2003). Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed. Official Journal of the European Union. 18 October; 268: 1–23.
- Environment Council of the European Union (2008). Genetically modified organisms – Council conclusions 16882/08. Brussels. 5 December.
- Arsenis, K., J. Bove, et al. (2012). Letter from MEPs to the Commission and member states on the draft GM food and feed guidelines. 9 January.
- Dalli J (2012). Letter to Members of the European Parliament. 3 March.
- European Parliament and Council (2001). Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC. Official Journal of the European Communities. 17 April: 1–38.





This is very disturbing news. We are fighting for GMO labeling in WA while the very purpose of the labeling is being diminished, if not eradicated, in the EU! The EU was (one of the) first to have labeling, and I thought it was going well. I was naïve to think BigAg. wouldn’t be a big player on the EU field. A ninety day trial is a very sad joke. They’ll come a day when only the 1%ers can afford to buy organic.